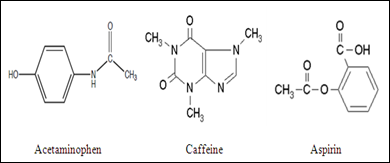
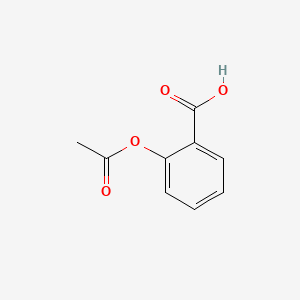
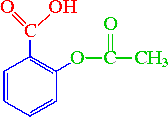
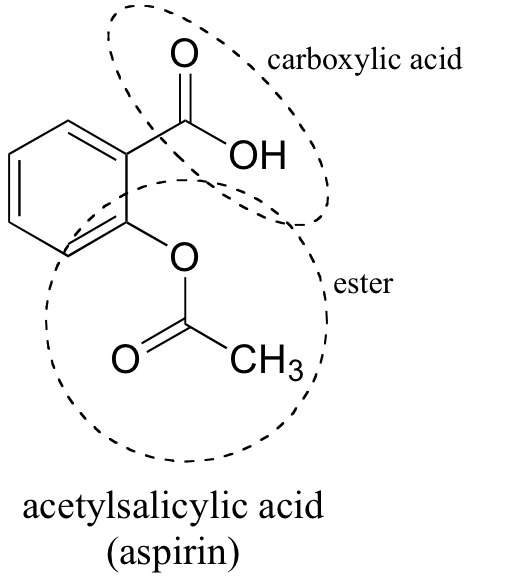
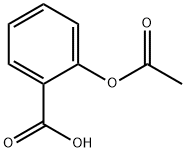
Between acetylsalicylic acid and benzoic acid, which is most polar if you look only at the structure (not at solubility in water g/l), and why? Why is that structure most polar? -
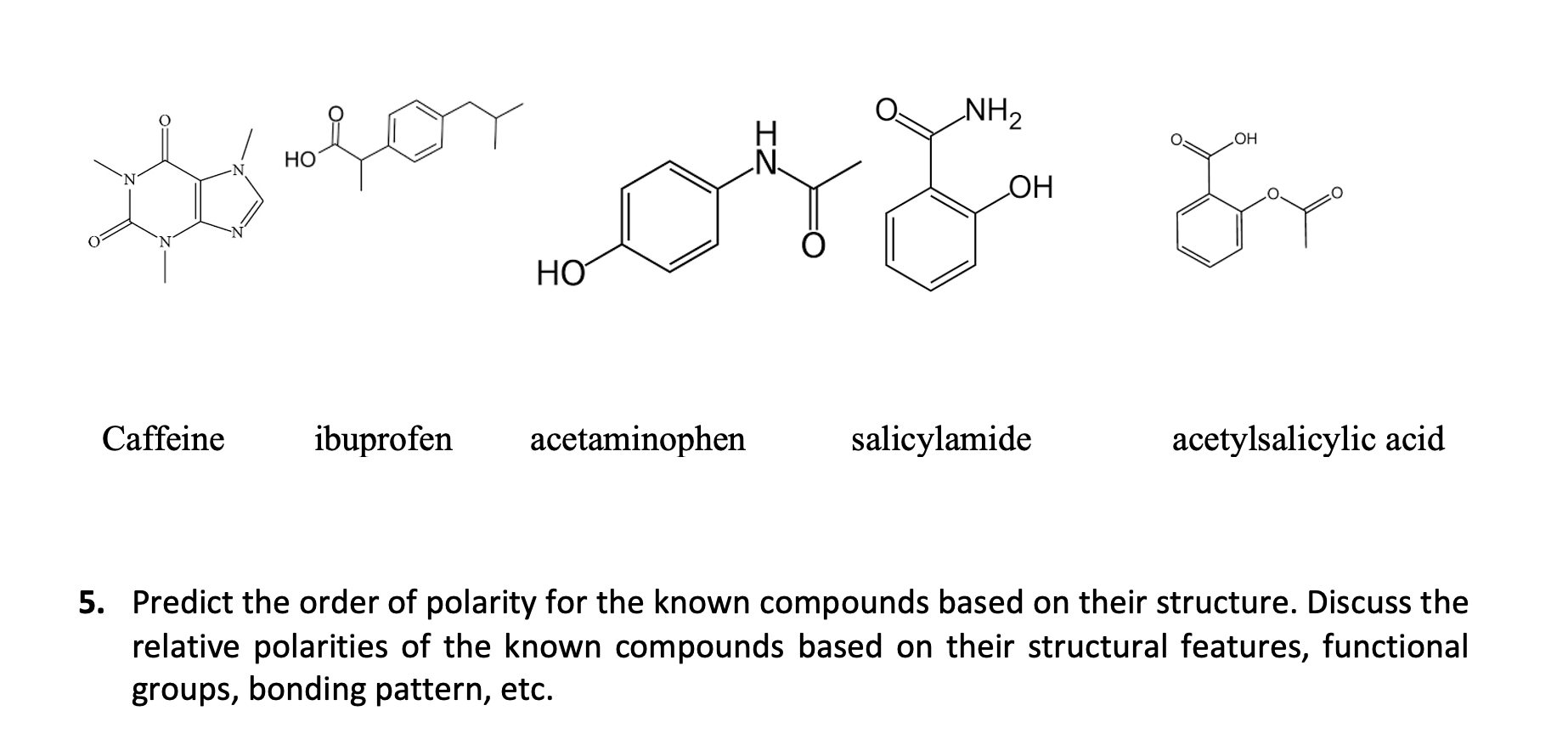
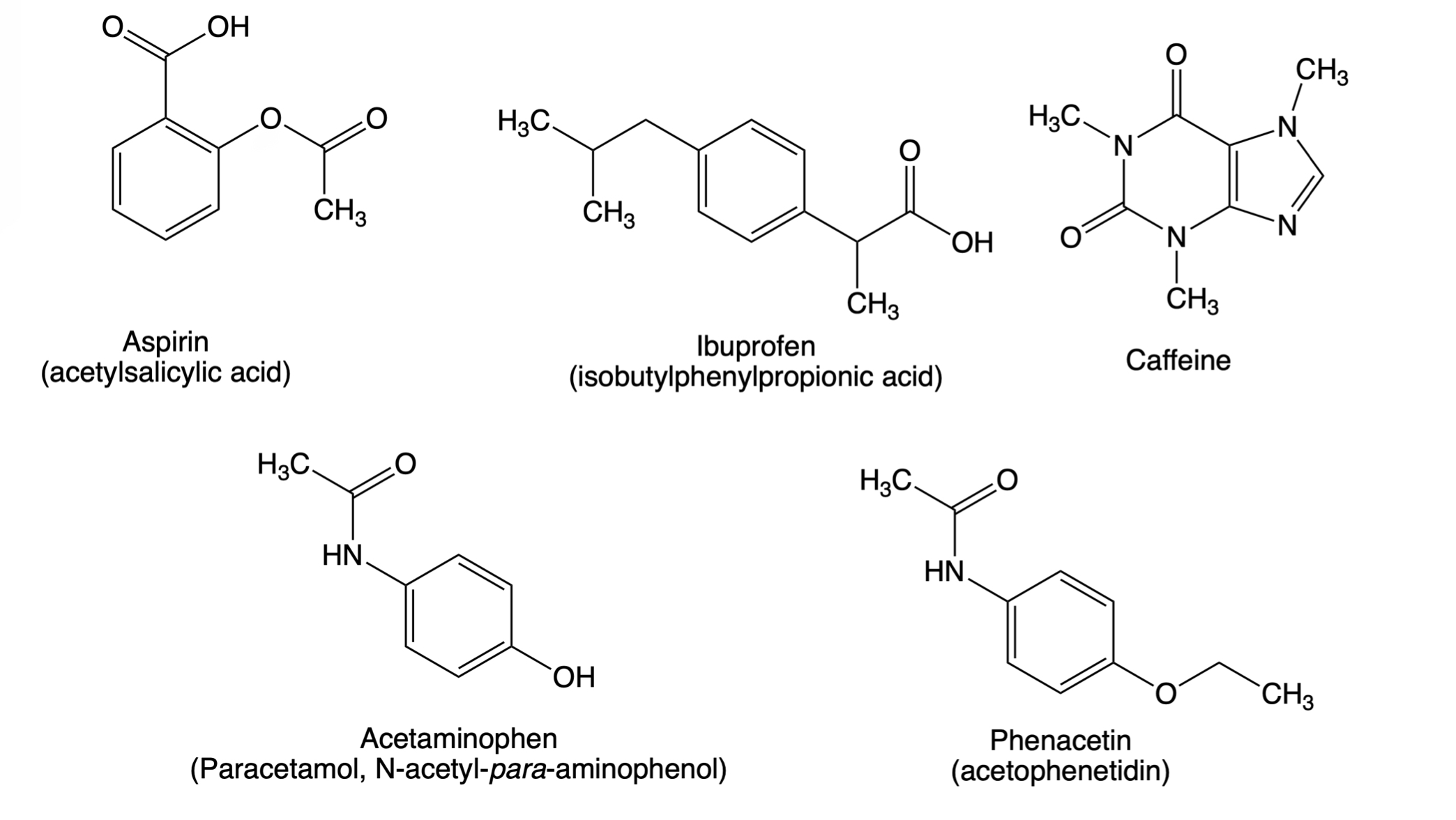
Why is Caffeine the most polar molecule on here when it doesn't have a single hydrogen bond? I've been taught that H-Bonds are more polar than any simple Diple-Dipole polarities that caffine

Is Aspirin Polar or Nonpolar? – (Polarity of Aspirin) | Molecular geometry, Molecular shapes, Organic molecules
Between acetylsalicylic acid and benzoic acid, which is most polar if you look only at the structure (not at solubility in water g/l), and why? Why is that structure most polar? -
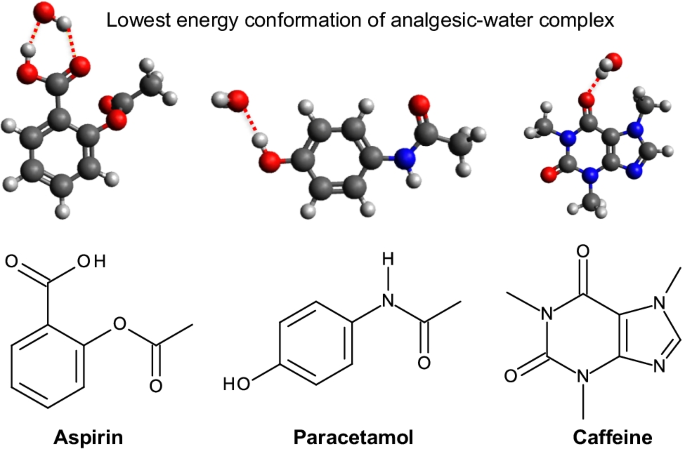
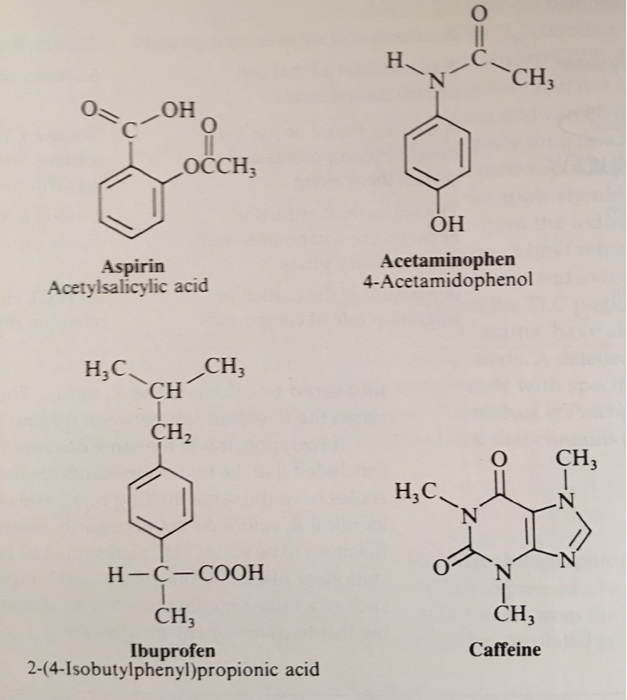
A DFT study of the interaction of aspirin, paracetamol and caffeine with one water molecule | Journal of Molecular Modeling

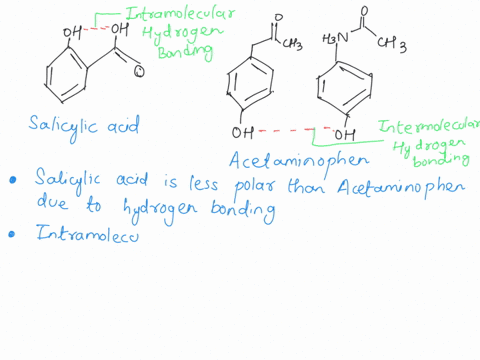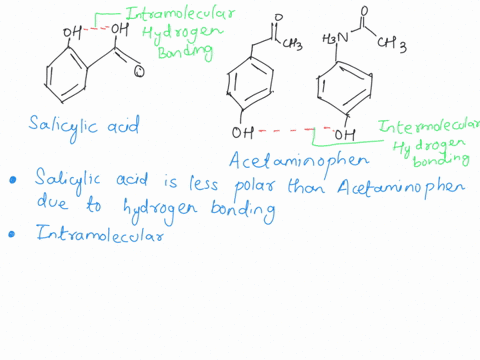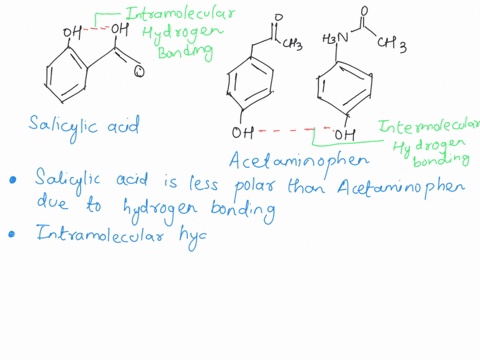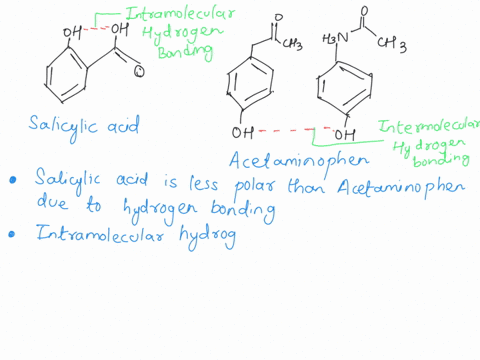
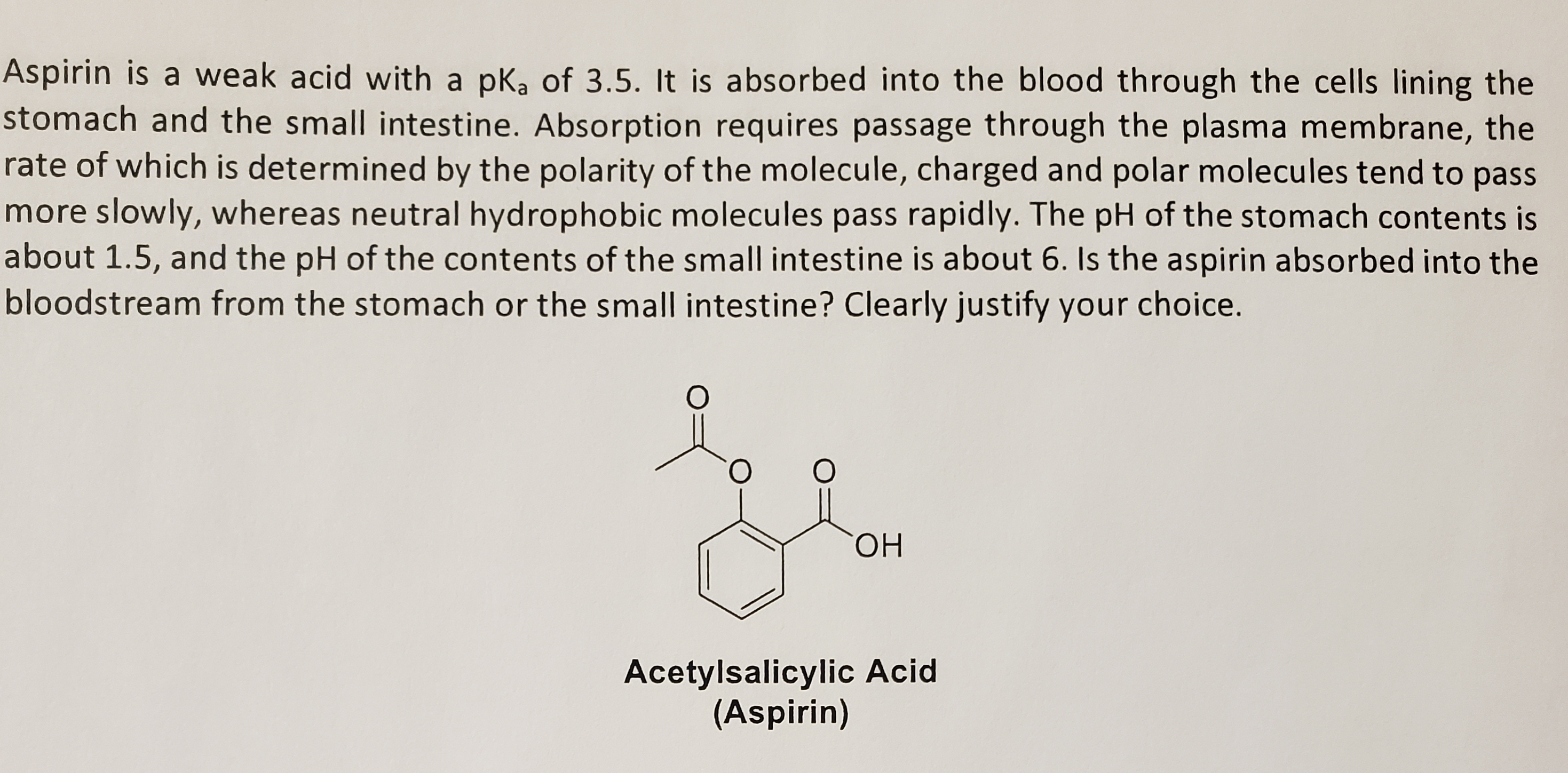
Why does Aspirin have a relatively small dipole moment, considering the presence of an acid and ester functional group?? | Homework.Study.com

Following is a molecular model of aspirin (acetylsalicylic acid). Identify the hybridization of the orbitals on each carbon atom in aspirin, and tell which atoms have lone pairs of electrons (gray =
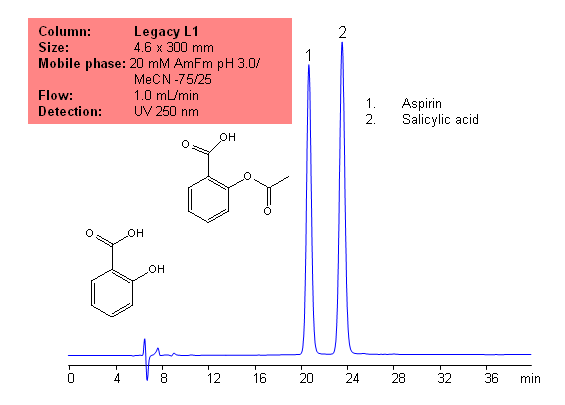
USP Methods for the Analysis of Aspirin (Acetylsalicylic acid (ASA)) Using Legacy L1 Column | SIELC Technologies